QUALITIDE
NMN (β-Nicotinamide Mononucleotide)
NMN (β-Nicotinamide Mononucleotide)
Couldn't load pickup availability
NMN (β-Nicotinamide mononucleotide) is a natural molecule produced by the body and is classified as a nucleotide. Nucleotides are involved in a wide array of important bodily functions, including as the building blocks of DNA. Within the cells, NMN is converted into another molecule called nicotinamide adenine dinucleotide (NAD+). NAD+ plays an integral role in energy production and regulation of vital cellular processes such as DNA repair, immune function, conversion of food into a usable form of energy called adenosine triphosphate (ATP), and regulation of circadian rhythm. In simple terms, NMN is the raw material and NAD+ is the refined version that the body can actually use to perform essential biological processes. In addition, the amount of NAD+ that the body can produce greatly depends on the available NMN.
NAD+ is not very bioavailable. This means that ingesting it directly will not achieve its therapeutic or desired effects. Therefore, one of the most effective ways of boosting NAD+ levels is through NMN supplementation.
Overall Health Benefits of NMN
Nicotinamide mononucleotide (NMN) supports overall health by extending lifespan, producing anti-aging effects, enhancing cognitive and cardiovascular function, improving metabolic health, boosting immunity, and promoting organ health, including the liver, kidneys, and eyes. It also helps combat inflammation, cancer, diabetes symptoms, and supports fertility, energy levels, and weight management.
- Extends lifespan [1-12]
- Produces anti-aging effects [13-25]
- Improves cognitive function [26-40]
- Lowers the risk of cardiovascular disease [41-55]
- Fights cancer [56-63]
- Improves blood sugar levels and treats diabetes symptoms [16, 64-74]
- Fights inflammation [75-78]
- Improves fertility [79-85]
- Improves eye health [86-100]
- Boosts immune function [14, 101-110]
- Increases energy levels [111-117]
- Promotes weight loss [118-120]
- Treats stroke [121-124]
- Improves liver health [125-132]
- Improves kidney health [133-137]
Key Takeaways
- NAD+ Booster: NMN is a precursor to nicotinamide adenine dinucleotide (NAD+), a vital coenzyme in cellular energy production. NAD+ levels decline with age, leading to reduced cellular function. Supplementing with NMN can help restore NAD+ levels, supporting overall health and potentially slowing aspects of aging.
- Anti-Aging Potential: Research suggests NMN may help mitigate age-related decline by promoting cellular repair, enhancing mitochondrial function, and improving metabolic processes. These benefits have made NMN a popular supplement in anti-aging and longevity circles.
- Metabolic Health Benefits: NMN supplementation has been associated with improvements in glucose metabolism, insulin sensitivity, and overall metabolic health, which may benefit conditions like obesity, type 2 diabetes, and other metabolic disorders.
- Cardiovascular and Neuroprotection: NMN shows promise in protecting cardiovascular health by supporting blood vessel function. Additionally, some studies suggest it may offer neuroprotective effects, helping to maintain cognitive function and potentially reduce the risk of neurodegenerative diseases.
- Safety and Dosage: NMN is generally considered safe at typical dosages used in studies (ranging from 250-500 mg daily). However, research is ongoing, and while early results are promising, long-term effects and optimal dosage need further exploration.
How NMN Works
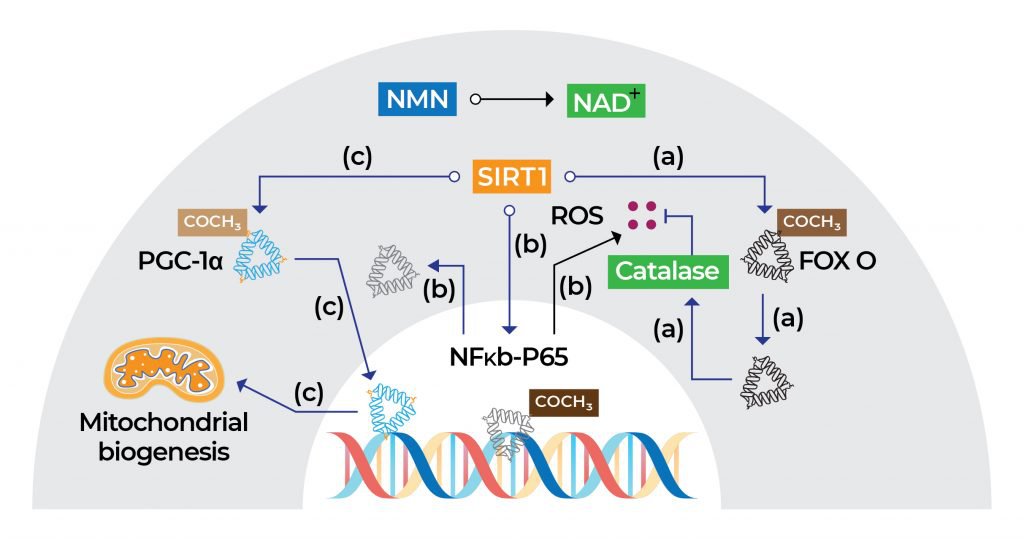
The health benefits of NMN can be attributed to its ability to boost NAD+ levels. Once NMN is converted into NAD+, activation of the sirtuin 1 (SIRT1) function in the nucleus of cells happens. SIRT1 is an enzyme that helps regulate proteins involved in cellular metabolism and processes associated with longevity, inflammation, and stress. In addition, the NMN-mediated increase in NAD+ levels counteracts age-related mitochondrial deterioration by promoting mitochondrial biogenesis, a process by which cells increase mitochondrial numbers.
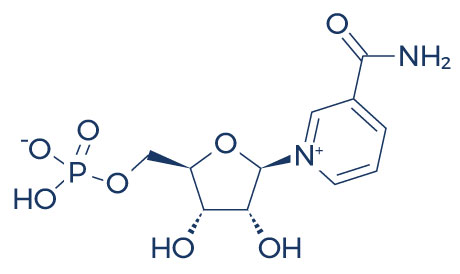
Chemical Structure of NMN
Research on NMN
A. Extends Lifespan
Within the cells, NMN is converted into NAD+ which plays an integral role in energy production and regulation of vital cellular processes. By boosting NAD+ levels, NMN can contribute to a longer lifespan. Studies show that people with higher NAD+ levels have a longer lifespan compared to those with lower NAD+ levels. [1-5]
Another mechanism that increases longevity is through increasing sirtuin (SIRT) activity which is associated with stable telomeres (located at chromosomes ends). This in turn helps attenuate the age-related telomere shortening which is linked to a shorter lifespan. [6] NMN boosts NAD+ levels which cause activation of SIRT, resulting in stable and longer telomeres. This process helps extends lifespan.
In addition, the NMN-mediated increase in NAD+ levels promotes mitochondrial biogenesis via SIRT1 activation. Mitochondrial biogenesis is characterized by the production of new mitochondria (the powerhouse of cells) and is essential for a longer lifespan since mitochondrial dysfunction is linked to various age-related diseases and a shorter lifespan. [7-8]
The longevity effects of NMN are backed by a number of studies:
- In mice, NMN maintained telomere length, reduced the DNA damage response, improved mitochondrial function, and rescued liver fibrosis (scarring) in a partially SIRT1-dependent manner. [9]
- Increasing sirtuin activity is known to stabilize telomeres and attenuate age-related telomere shortening. [10] Since the NMN-mediated increase in NAD+ activates SIRT1, it can help achieve chromosome stability and longer telomeres.
- In a rodent model of decompensated hemorrhagic shock, rats that received NMN had decreased inflammation, improved cellular metabolism, and increased survival. [11]
- In mice with progressive neurodegeneration, the addition of NMN in the drinking water of the subjects normalized neuromuscular function, delayed memory loss, and extended lifespan. [12]
B. Anti-Aging Effects
Mitochondrial aging contributes to cellular senescence (also known as biological aging), increased inflammation, decreased stem cell activity, reduced healing rate, and a decline in tissue and organ function. [13] Interestingly, studies show that the NMN-mediated increase in NAD+ levels produces anti-aging effects such as increasing mitochondrial numbers, amelioration of mitochondrial dysfunction, and promotion of chromosome stability via activation of sirtuin 1 (SIRT1), stimulation of DNA repair, and maintaining telomere length:
- The administration of NMN via injections in elderly mice reversed age-related mitochondrial deterioration. It was observed that declining NAD+ levels were associated with interruptions in the normal signaling between the cell’s nucleus and mitochondria and that raising NAD+ levels via NMN administration restored the communication between these cellular structures. [14]
- In elderly mice, treatment with NMN improved blood flow and increased endurance via the promotion of SIRT1-dependent increases in capillary density. [15]
- In mice, the administration of NMN prevented age-related weight gain and improved physical activity, energy metabolism, lipid profiles, and insulin sensitivity. [16]
- In healthy men, the single oral administration of NMN was safe and effectively metabolized without any adverse effects, indicating a potential therapeutic strategy to mitigate disorders related to aging. [17-18]
- NMN effectively mitigated the age-associated physiological decline in the lungs of old mice and bleomycin-induced pulmonary fibrosis in young mice. [19]
- In aged mice, NMN treatment promoted mitochondrial rejuvenation and decreased inflammation. [20]
- In pre-aging male mice, oral short-term administration of NMN significantly increased telomere length. [21]
- Elevating NAD+ levels has been shown to slow down various mechanisms associated with aging such as decreased energy production in the mitochondria, oxidative stress, DNA damage, cognitive impairment, and inflammation. [22-23]
- NMN has been shown to slow down age-related changes in the skin by restoring skin homeostasis, protecting against oxidative stress, increasing mitochondrial efficiency, and reducing excess melanin production. [24-25]
C. Improves Cognitive Function
A decline in NAD+ levels is associated with brain disorders such as Alzheimer’s disease, Parkinson’s disease, and other conditions that cause cognitive impairment. [26] By boosting NAD+ levels, NMN can lower the risk for these medical conditions. Another interesting mechanism is that the NMN-mediated increase in NAD+ levels can decrease the production of reactive oxygen species (ROS), which are linked to various brain disorders. Moreover, NMN can also help reverse the age-related cognitive decline by mitigating mitochondrial dysfunction.
A number of studies demonstrate the beneficial effects of NMN on cognitive function:
- In an Alzheimer’s disease-relevant murine model, NMN treatment restored mitochondrial respiratory function in the brain. [27]
- In an animal model of Alzheimer’s disease, NMN treatment significantly decreased the production of β-amyloid (abnormal protein structures), loss of nerve signaling, and inflammatory response. [28]
- In a rat model of vascular cognitive impairment, NMN protected against cognitive decline. [29]
- In older rats, NMN treatment at a dose of 100 mg/kg alleviated aging-induced memory impairment via modulation of mitochondrial function and apoptosis (programmed cell death) in the brain. [30]
- In D-galactose-induced aging rat models, the combination of NMN and lycopene improved the ability of spatial location learning and memory. [31]
- In rats, NMN ameliorated neuronal damage and cognitive impairment caused by severe hypoglycemia (low blood sugar levels). [32]
- In rats, NMN protected against diabetes-induced memory deficits by preserving mitochondrial oxidative phosphorylation (OXPHOS) function and preventing neuronal loss. [33]
- In the brain cells of aged rats, NMN treatment increased the formation of new blood vessels and decreased the production of oxidative stress. [34]
- In a rat model of Alzheimer’s disease, NMN protected against β-amyloid oligomer-induced cognitive impairment and neuronal death. [35]
- In aged mice, NMN supplementation improved cognitive function by ameliorating age-related cerebromicrovascular dysfunction. [36]
- Studies found that NMN can help improve cognitive function by promoting the renewal of neural stem/progenitor cells (NSPCs) via SIRT1, SIRT2, and SIRT6. [37-38]
- In old mice, short-term NMN supplementation improved the sensory processing aspect of some aversive stimuli, suggesting that the treatment can treat cognitive impairments and enhance the quality of life in old age. [39]
- In a cellular model of Parkinson’s disease (PD), ameliorated mitochondrial inhibitor-induced impairments of energy metabolism and inhibited death of brain cells. [40]
D. Lowers the Risk of Cardiovascular Disease
The NMN-mediated increase in NAD+ levels activates SIRT1, which in turn increases the production of cardioprotective molecules, such as MnSOD (antioxidants), Trx1 (antioxidants), and Bcl-xL (anti-apoptotic). [41] In addition, SIRT1 activation can also help protect the heart from inflammation and oxidative stress.
Compelling evidence supports the cardioprotective effects of NMN:
- In mice, NMN protected against heart injury caused by insufficient blood flow (ischemia). [42]
- A study suggests that NMN exerts its cardioprotective effects by generating adenosine triphosphate via glucose breakdown. [43]
- In mice with ischemia, NMN (62.5mg/kg) dramatically ameliorated injury and significantly improved the neurological outcome. [44]
- In mice, NMN treatment prevented post-ischemic depletion of mitochondrial NAD+, suppressed mitochondrial fragmentation, and reduced oxidative stress via SIRT3-dependent mechanisms. [45-46]
- In the heart cells of mice, short-term administration of NMN preserved mitochondrial ultrastructure, reduced oxidative stress, and prevented cell death in the heart. [47]
- Studies reported that NMN administration in patients with intractable cardiac diseases such as heart failure with preserved ejection fraction may produce beneficial effects. [48-49]
- In rats, NMN attenuated doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation, and programmed cell death. [50]
- In aged male rats, NMN counteracted damage to the heart muscle by activating SIRT3/FOXO1 and reducing programmed cell death. [51]
- In mice with heart scarring, NMN administration via injections reduced scarring by suppressing oxidative stress and Smad3 acetylation in a NAD+/SIRT1-dependent manner. [52]
- In aged mice, NMN administration increased NAD+ levels and protected against ischemic heart injury. [53-55]
E. Fights Cancer
Mitochondrial respiration malfunction and increased glucose uptake are mechanisms observed in cancer cells. [56] The NMN-mediated increase in NAD+ levels has been shown to increase mitochondrial respiration and reduce glucose (blood sugar) uptake, indicating that NMN may help combat cancer. Another important mechanism is that NMN increases NAD+ levels which in turn activates SIRT1 and SIRT6, both of which inhibit the growth and spread of tumors.
A number of studies support the anti-cancer properties of NMN:
- The NMN-mediated increase in NAD+ levels is associated with cell cycle arrest and programmed cell death of malignant cells, enhanced efficacy of chemotherapeutic drugs and radiation therapy, and prevention of cancer cell progression. [57-61]
- NMN has been shown to combat cancer by boosting cellular energy and enhancing DNA repair activity. [62]
- NMN has also been shown to enhance colorectal cancer cell-kill by the chemotherapeutic drug Tiazofurin. [63]
F. Improves Blood Sugar Levels and Treats Diabetes Symptoms
NMN has the ability to improve the body’s response to the hormone insulin, which helps blood sugar enter the cells. This process is called insulin sensitivity. With increased insulin sensitivity, blood sugar stays at healthy levels.
The blood sugar-lowering effects of NMN and its benefits on diabetes symptoms are backed by a number of studies:In mice, the administration of NMN prevented age-related weight gain and improved physical activity, energy metabolism, lipid profiles, and insulin sensitivity. [16]
- In prediabetic women, NMN supplementation at 250 mg/day increased muscle insulin sensitivity. [64]
- In obese mice, increased NAD+ levels induced by NMN improved blood glucose and lipid homeostasis by increasing the activity of SIRT1 and SIRT3. [65-66]
- In old mice with type 2 diabetes, NMN improved glucose intolerance and lipid profiles. [67]
- In mice, NMN treatment ameliorated NAD+ deficiency and improved insulin secretion. [68]
- In mice fed with fructose, a type of sugar, administration of NMN restored insulin secretion by correcting inflammation of the islet of the pancreas (responsible for insulin production). [69]
- Studies found that NMN supplementation for 12 months decreased insulin resistance in mice. [70-71]
- In mice fed with a high-fat diet, NMN administered via intravenous injections improved glucose tolerance. [72]
- A cell study found that NMN can stimulate insulin secretion. [73]
- In lean type 2 diabetic patients with secondary failure to sulphonylureas (anti-diabetic medication), NMN improved insulin secretion and metabolic control. [74]
G.Fights Inflammation
NMN has the potential to suppress inflammaging, which is the age-related increase in inflammation. Specifically, NMN has been found to suppress cyclooxygenase-2 (COX-2), an enzyme that synthesizes the proinflammatory mediators known as prostaglandins. With this effect, NMN can help treat and ward off a wide array of inflammatory conditions.
A convincing number of studies support the anti-inflammatory effects of NMN:
- In mice, NMN inhibited lipopolysaccharide (LPS)-induced inflammation and oxidative stress via suppression of COX-2. [75]
- In aging mice, NMN reduced inflammatory markers such as tumor necrosis factor alpha (TNF-α). [76]
- In mice with inflammation of the abdomen due to blood infection, NMN prevented clinical deterioration and improved survival. [77]
- A cell study found that NMN inhibited endothelial inflammation and improved the function of nitric oxide (a substance that widens the blood vessels). [78]
H. Improves Fertility
NMN has the capacity to improve male and female fertility. It does this by improving the quality of both the egg cell and sperm cell. This in turn ensures successful fertilization and pregnancy. In addition, NMN can also help reverse some of the effects of aging on the reproductive system.
The beneficial effects of NMN on male and female reproductive health are backed by a number of studies:
- In animal subjects, NMN supplementation protected egg cell quality against environmental pollutant-induced deterioration, contributing to improved fertility. [79]
- In aged animals, treatment with the NAD+ metabolic precursor NMN rejuvenated egg cell quality, leading to the restoration of fertility. [80-81]
- NMN supplementation improved the quality of porcine egg cells under heat stress by restoring cell division. [82]
- Supplementation of NMN improved the quality of postovulatory aged porcine egg cells. [83]
- In female mice, NMN supplementation improved egg cell quality by restoring mitochondrial structures. [84]
- In streptozotocin-induced diabetic mice, NMN treatment significantly increased the area and diameter of seminiferous tubules and the number of spermatogenic cells and sperms. [85]
I. Improves Eye Health
Restoration of NAD+ through NMN supplementation can help protect photoreceptors (special cells in the retina that converts light into signals that are sent to the brain) against light-induced retinal damage. [86-87] The exact mechanism of NMN-induced eye protection can be attributed to SIRT1 activation since it is essential in the development and survival of the retina. Alterations in SIRT1 activity have been linked to various eye conditions such as aged retina, diabetic retinopathy, light-induced retinal degeneration, and oxygen-induced retinal damage. [88-93]
Studies show that NMN supplementation is essential for eye health:
- NMN treatment increased NAD+ levels and improved cell viability, reduced programmed cell death, and decreased lactate dehydrogenase (LDH) release in corneal epithelial cells. [94]
- In high-glucose-treated human corneal epithelial cells, NMN increased cell viability by reversing cell damage, reducing programmed cell death, increasing cell migration, and restoring the structures of corneal cells. [95]
- A study reported that NMN supplementation can treat glaucoma and age-related macular degeneration by correcting NAD+ pool depletion and mitochondrial dysfunction. [96]
- In a mouse model of retinal ischemia-reperfusion injury (cellular dysfunction and death after the restoration of blood flow to tissues with previously impaired blood circulation), NMN injection significantly suppressed retinal functional damage and inflammation and protected against oxidative stress-induced cell death. [97]
- In a photoreceptor degenerative model of retinal detachment, NMN administration exerted neuroprotective effects on photoreceptors and against oxidative injury. [98]
- In mice, NMN effectively prevented ultraviolet B light-induced tissue damage and endothelial cell death in the mouse cornea. [99]
- In mice with corneal injury, the replenishment of NMN or NAD+ slowed down corneal nerve fiber degeneration by restoring the activation levels of SIRT1. [100]
J. Boosts Immune Function
The age-related shortening of telomeres adversely affects immune function, thus, increasing the risk of severe infection, inflammatory conditions, and chronic diseases. [101-103] Interestingly, NMN boosts NAD+ levels which in turn activates SIRT1. As a result, the telomeres lengthen and become more stable. Moreover, NMN has anti-inflammatory effects and the ability to regulate the activity of certain cells of the immune system.
A good deal of evidence supports the immune-boosting effects of NMN:
- Treatment of 24-month-old mice with NMN for 1 week significantly reduced the levels of inflammatory markers such as TNFα and IL-6 in the skeletal muscle. [14]
- In young and older mice, NMN augmented the cytotoxic activity of natural killer cells of the immune system. [104]
- The NMN-mediated increase in NAD+ levels can help improve immune function by promoting cell survival, DNA repair, and enhanced intercellular communication. [105-106]
- Restoring normal NAD+ levels via NMN can decrease the severity of immune reactions in patients with COVID-19 infection. [107]
- An increase in NAD+ levels was associated with significant immunomodulatory effects such as modulation of cytokine action, regulation of the intercellular adhesion molecules, blockage of mast cell degranulation, and inhibition of protease release from leukocytes. [108-110]
K. Increases Energy Levels
Sirtuins play a critical role in regulating various cellular functions including energy metabolism, stress resistance, and circadian rhythm neuronal function – all of which are essential for increasing energy levels. [111-112] Since NMN activates SIRT1 by increasing NAD+ levels, it may help boost energy levels and reduce fatigue. Moreover, NAD+ is essential for the production of adenosine triphosphate (ATP), which is needed by the cells to perform various biological functions.
An increasing number of studies support the beneficial effects of NMN on energy levels and medical conditions that cause fatigue:
- In older adults, NMN intake in the afternoon for 12 weeks effectively improved sleep quality, fatigue, and physical performance as evidenced by improved lower limb function and reduced drowsiness. [113]
- In amateur runners, NMN supplementation for 6 weeks increased the aerobic capacity during exercise training via enhanced O2 utilization of the skeletal muscle. [114]
- In healthy young and elderly mice, NMN supplementation at 500 mg/kg/d with exercise training increased endurance performance. [115-116]
- Raising intracellular NAD+ levels through NMN supplementation can improve the quality of life of patients with chronic fatigue syndrome by improving neurological function, promoting energy production, and lowering fatigue. [117]
L. Promotes Weight Loss
NMN can help promote weight loss via different mechanisms such as increased energy expenditure and enhanced insulin sensitivity. Increased energy expenditure prevents excess fat storage. With enhanced insulin sensitivity, the body responds well to the effects of insulin which in turn prevents high blood sugar (hyperglycemia) which is associated with increased adiposity.
Evidence suggests that NMN is beneficial for achieving a healthier weight because of its fat-burning properties:
- In healthy individuals, the intravenous administration of NMN significantly reduced blood triglyceride (TG) levels and fat accumulation in the liver. [118]
- In mice with severe insulin resistance, NMN treatment reduced visceral adipose tissue (VAT) and adiponectin (a hormone produced by fat cells). [119]
- In obese female mice, NMN reduced adiposity and improved glucose and markers of mitochondrial function. [120]
M. Treats Stroke
A stroke occurs when the blood supply to the brain is cut off. NMN has the ability to widen the blood vessels which can help restore blood flow to the brain. In addition, the anti-inflammatory effects of NMN can help relieve brain swelling associated with stroke.
A number of studies suggest that NMN treatment is beneficial in treating the symptoms of stroke and improving recovery outcomes:
- In mice with brain injury caused by stroke, NMN treatment for 7 days markedly promoted the recovery of body weight and neurological function via suppression of brain inflammation and oxidative stress. [121]
- In a rodent model of hemorrhagic shock due to stroke, NMN significantly improved survival after resuscitation. [122]
- NAD replenishment with NMN protected blood-brain barrier integrity and attenuated brain changes caused by significant bleeding. [123]
- NMN treatment attenuated traumatic brain injury in mice via restoration of NAD+ levels. [124]
N. Improves Liver Health
NMN boosts NAD+ levels resulting in SIRT1 activation. This process is essential in liver health as SIRT1 activation improves cholesterol, fat, and lipid transport as well as fatty acid homeostasis in the liver. [125-127]
Studies show that NMN can improve liver function and protect against liver disease:
- NMN treatment can help protect against liver injury by raising NAD+ levels. [128]
- An increase in NAD+ has been shown to protect against aging-induced non-alcoholic fatty liver disease-like liver dysfunction in mice. [129]
- Increased NAD+ levels can prevent the progression of non-alcoholic fatty liver disease by influencing the oxidative stress response, programmed cell death, and inflammatory response. [130]
- In mouse models of liver cirrhosis (scarring), NMN treatment inhibited the production of substances that cause liver inflammation and scarring. [131]
- In aged mice, NMN administration protected against oxidative stress-induced liver injury. [132]
O. Improves Kidney Health
The anti-aging effects of NMN can also help address the age-related decline in kidney function. Reduced levels of NAD+ are associated with reduced sirtuin activity which in turn causes deterioration in the overall function of the kidneys. The ability of NMN to boost NAD+ levels activates SIRT1 which can possibly mitigate the negative effects of aging on the kidneys.
Evidence suggests that NMN can help address kidney problems associated with aging and certain medical conditions:
- In old mice with acute kidney injury, NMN supplementation improved kidney function via restoration of renal SIRT1 activity and NAD+ content. [133]
- In human kidney cells, NMN suppressed DNA damage and senescence induced by hydrogen peroxide and hypoxia (low oxygen). [134]
- In mice, short-term NMN treatment ameliorated adriamycin-induced kidney damage by increasing SIRT1. [135]
- In mice with kidney complications due to diabetes, NMN treatment increased kidney concentrations of NAD+ and SIRT1, improved survival rates, and alleviated kidney scarring. [136-137]
Nicotinamide Mononucleotide Side Effects
NMN side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on NMN. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of NMN. Despite this, it was listed as a side effect associated with NMN even though these associated side effects are very uncommon.
Side effects associated with NMN may include the following:
- Abdominal distension
- Abdominal pain
- Belching
- Diarrhea
- Fatigue
- Fever
- Flatus
- Joint pain
- Muscle pain
- Sense of hunger
What is NMN Supplement (Nicotinamide Mononucleotide Supplement)?
Nicotinamide Mononucleotide (NMN) is a molecule that occurs naturally in the body and plays a crucial role in cellular metabolism. It is a precursor to nicotinamide adenine dinucleotide (NAD+), a vital coenzyme involved in numerous biological processes, including energy production, DNA repair, and cellular aging. NMN supplements aim to boost NAD+ levels, which tend to decline with age, potentially supporting overall health and longevity.
Research into NMN supplements has been promising, suggesting they may have various health benefits. Studies in animals have indicated that NMN can improve metabolic health, enhance physical activity, and slow down certain aspects of aging. In humans, preliminary research suggests that NMN supplementation may help improve insulin sensitivity, increase muscle strength, and support cardiovascular health, although more extensive clinical trials are needed to fully understand its effects.
Despite the potential benefits, NMN supplements should be approached with caution. The supplement industry is not strictly regulated, so the quality and effectiveness of NMN products can vary. It’s important to consult with a healthcare professional before starting any new supplement regimen, especially if you have underlying health conditions or are taking other medications.
Nicotinamide Mononucleotide vs Nicotinamide Riboside (NMN vs NR)?
Nicotinamide Mononucleotide (NMN) and Nicotinamide Riboside (NR) are both compounds that play a role in the production of NAD+ (nicotinamide adenine dinucleotide), a vital coenzyme involved in numerous cellular processes, including energy metabolism and DNA repair. NMN is a direct precursor to NAD+, meaning it is converted into NAD+ more directly within cells. This pathway potentially makes NMN a more efficient option for boosting NAD+ levels.
On the other hand, Nicotinamide Riboside (NR) is a slightly different compound that also contributes to NAD+ synthesis but through a more indirect route. NR is first converted into nicotinamide mononucleotide (NMN) before being transformed into NAD+. This extra step may influence its effectiveness compared to NMN. However, research suggests that NR is still highly effective in increasing NAD+ levels and has demonstrated various health benefits in studies.
Both NMN and NR have shown promise in preclinical and clinical studies for their potential anti-aging effects, including improving metabolic health and enhancing physical endurance. While both compounds seem to offer similar benefits, the choice between NMN and NR might come down to individual preferences or specific health goals. Ongoing research continues to explore their comparative efficacy and optimal use.
What is NMN Powder?
NMN powder is a dietary supplement derived from nicotinamide mononucleotide (NMN), a naturally occurring compound in the body that plays a crucial role in cellular metabolism. NMN is a precursor to nicotinamide adenine dinucleotide (NAD+), a coenzyme essential for energy production, DNA repair, and various metabolic processes. As we age, NAD+ levels decline, which can impact overall health and vitality.
Supplementing with NMN powder is believed to help boost NAD+ levels, potentially counteracting some effects of aging and supporting cellular function. Research into NMN’s benefits is ongoing, but preliminary studies suggest that it may enhance physical endurance, improve cognitive function, and promote healthier aging by improving cellular energy production and repair mechanisms.
NMN powder is typically taken as a dietary supplement in capsule or powdered form. While promising, it’s important to approach NMN with a balanced perspective, as more research is needed to fully understand its long-term effects and benefits. Consulting with a healthcare provider before starting any new supplement regimen is recommended to ensure it aligns with individual health needs and goals.
What is NMN Sublingual?
NMN sublingual refers to nicotinamide mononucleotide (NMN) delivered via a sublingual method, meaning it is taken under the tongue. NMN is a compound that plays a crucial role in the production of NAD+ (nicotinamide adenine dinucleotide), a coenzyme involved in various biological processes, including energy metabolism and cellular repair. By delivering NMN directly under the tongue, the supplement can be absorbed more rapidly into the bloodstream, bypassing the digestive system and potentially increasing its effectiveness.
The sublingual form of NMN is designed to offer faster absorption and higher bioavailability compared to oral tablets or capsules. This method leverages the rich blood supply under the tongue, which allows for quicker entry into the systemic circulation. Consequently, users might experience more immediate effects and enhanced benefits related to NMN’s role in promoting cellular health and combating age-related decline.
Many proponents of NMN sublingual supplements believe they can contribute to improved energy levels, cognitive function, and overall vitality. Research into NMN’s potential benefits is ongoing, but preliminary studies suggest that enhancing NAD+ levels may have positive effects on aging and various health conditions. As with any supplement, it is important to consult with a healthcare provider before starting NMN sublingual to ensure it is appropriate for individual health needs and conditions.
NMN Body Building
Nicotinamide Mononucleotide (NMN) has gained attention in the bodybuilding community for its potential benefits in enhancing physical performance and recovery. NMN is a precursor to Nicotinamide Adenine Dinucleotide (NAD+), a vital coenzyme involved in cellular energy production and metabolism. By boosting NAD+ levels, NMN may improve muscle endurance, reduce fatigue, and promote more efficient recovery after intense workouts.
Research into NMN’s impact on bodybuilding is still emerging, but some studies suggest it could help mitigate age-related declines in muscle function and strength. As we age, NAD+ levels naturally decrease, which can contribute to decreased muscle mass and performance. Supplementing with NMN might counteract these effects, helping bodybuilders maintain their muscle mass and strength over time.
Additionally, NMN’s potential anti-inflammatory and antioxidant properties could offer further advantages for bodybuilders. Reducing oxidative stress and inflammation can help in preventing exercise-induced muscle damage and speeding up recovery. While more research is needed to fully understand NMN’s effects, its role in supporting cellular health makes it an intriguing option for those looking to enhance their bodybuilding regimen.
Reference
-
Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004 May 14;117(4):495-502. doi: 10.1016/s0092-8674(04)00416-7. PMID: 15137942.
-
Yang, N. C., Cho, Y. H., & Lee, I. (2019). The Lifespan Extension Ability of Nicotinic Acid Depends on Whether the Intracellular NAD+ Level Is Lower than the Sirtuin-Saturating Concentrations. International journal of molecular sciences, 21(1), 142. https://doi.org/10.3390/ijms21010142.
-
Hashimoto, T., Horikawa, M., Nomura, T., & Sakamoto, K. (2010). Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology, 11(1), 31–43. https://doi.org/10.1007/s10522-009-9225-3.
-
Rajman, L., Chwalek, K., & Sinclair, D. A. (2018). Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell metabolism, 27(3), 529–547. https://doi.org/10.1016/j.cmet.2018.02.011.
-
Yaku, K., Okabe, K., & Nakagawa, T. (2018). NAD metabolism: Implications in aging and longevity. Ageing research reviews, 47, 1–17. https://doi.org/10.1016/j.arr.2018.05.006.
-
Palacios, J. A., Herranz, D., De Bonis, M. L., Velasco, S., Serrano, M., & Blasco, M. A. (2010). SIRT1 contributes to telomere maintenance and augments global homologous recombination. The Journal of cell biology, 191(7), 1299–1313. https://doi.org/10.1083/jcb.201005160.
-
Wang, Y., Oxer, D., & Hekimi, S. (2015). Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nature communications, 6, 6393. https://doi.org/10.1038/ncomms7393.
-
Lanza, I. R., & Nair, K. S. (2010). Mitochondrial function as a determinant of life span. Pflugers Archiv: European journal of physiology, 459(2), 277–289. https://doi.org/10.1007/s00424-009-0724-5.
-
Amano, H., Chaudhury, A., Rodriguez-Aguayo, C., Lu, L., Akhanov, V., Catic, A., Popov, Y. V., Verdin, E., Johnson, H., Stossi, F., Sinclair, D. A., Nakamaru-Ogiso, E., Lopez-Berestein, G., Chang, J. T., Neilson, J. R., Meeker, A., Finegold, M., Baur, J. A., & Sahin, E. (2019). Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell metabolism, 29(6), 1274–1290.e9. https://doi.org/10.1016/j.cmet.2019.03.001.
-
Sims, C. A., Guan, Y., Mukherjee, S., Singh, K., Botolin, P., Davila, A., Jr, & Baur, J. A. (2018). Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock. JCI insight, 3(17), e120182. https://doi.org/10.1172/jci.insight.120182.
-
Fang, E. F., Kassahun, H., Croteau, D. L., Scheibye-Knudsen, M., Marosi, K., Lu, H., Shamanna, R. A., Kalyanasundaram, S., Bollineni, R. C., Wilson, M. A., Iser, W. B., Wollman, B. N., Morevati, M., Li, J., Kerr, J. S., Lu, Q., Waltz, T. B., Tian, J., Sinclair, D. A., Mattson, M. P., … Bohr, V. A. (2016). NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell metabolism, 24(4), 566–581. https://doi.org/10.1016/j.cmet.2016.09.004.
-
Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., D’Amico, D., Ropelle, E. R., Lutolf, M. P., Aebersold, R., Schoonjans, K., Menzies, K. J., & Auwerx, J. (2016). NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (New York, N.Y.), 352(6292), 1436–1443. https://doi.org/10.1126/science.aaf2693.
-
Sun, N., Youle, R. J., & Finkel, T. (2016). The Mitochondrial Basis of Aging. Molecular cell, 61(5), 654–666. https://doi.org/10.1016/j.molcel.2016.01.028.
-
Gomes, A. P., Price, N. L., Ling, A. J., Moslehi, J. J., Montgomery, M. K., Rajman, L., White, J. P., Teodoro, J. S., Wrann, C. D., Hubbard, B. P., Mercken, E. M., Palmeira, C. M., de Cabo, R., Rolo, A. P., Turner, N., Bell, E. L., & Sinclair, D. A. (2013). Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell, 155(7), 1624–1638. https://doi.org/10.1016/j.cell.2013.11.037.
-
Das, A., Huang, G. X., Bonkowski, M. S., Longchamp, A., Li, C., Schultz, M. B., Kim, L. J., Osborne, B., Joshi, S., Lu, Y., Treviño-Villarreal, J. H., Kang, M. J., Hung, T. T., Lee, B., Williams, E. O., Igarashi, M., Mitchell, J. R., Wu, L. E., Turner, N., Arany, Z., … Sinclair, D. A. (2018). Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell, 173(1), 74–89.e20. https://doi.org/10.1016/j.cell.2018.02.008.
-
Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., Redpath, P., Migaud, M. E., Apte, R. S., Uchida, K., Yoshino, J., & Imai, S. I. (2016). Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell metabolism, 24(6), 795–806. https://doi.org/10.1016/j.cmet.2016.09.013.
-
Irie, J., Inagaki, E., Fujita, M., Nakaya, H., Mitsuishi, M., Yamaguchi, S., Yamashita, K., Shigaki, S., Ono, T., Yukioka, H., Okano, H., Nabeshima, Y. I., Imai, S. I., Yasui, M., Tsubota, K., & Itoh, H. (2020). Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocrine journal, 67(2), 153–160. https://doi.org/10.1507/endocrj.EJ19-0313.
-
Okabe, K., Yaku, K., Uchida, Y., Fukamizu, Y., Sato, T., Sakurai, T., Tobe, K., & Nakagawa, T. (2022). Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects. Frontiers in nutrition, 9, 868640. https://doi.org/10.3389/fnut.2022.868640.
-
Fang, T., Yang, J., Liu, L., Xiao, H., & Wei, X. (2021). Nicotinamide mononucleotide ameliorates senescence in alveolar epithelial cells. MedComm, 2(2), 279–287. https://doi.org/10.1002/mco2.62.
-
Kiss, T., Nyúl-Tóth, Á., Balasubramanian, P., Tarantini, S., Ahire, C., Yabluchanskiy, A., Csipo, T., Farkas, E., Wren, J. D., Garman, L., Csiszar, A., & Ungvari, Z. (2020). Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. GeroScience, 42(2), 527–546. https://doi.org/10.1007/s11357-020-00165-5.
-
Niu, K. M., Bao, T., Gao, L., Ru, M., Li, Y., Jiang, L., Ye, C., Wang, S., & Wu, X. (2021). The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase. Frontiers in nutrition, 8, 756243. https://doi.org/10.3389/fnut.2021.756243.
-
Nadeeshani, H., Li, J., Ying, T., Zhang, B., & Lu, J. (2021). Nicotinamide mononucleotide (NMN) as an anti-aging health product – Promises and safety concerns. Journal of advanced research, 37, 267–278. https://doi.org/10.1016/j.jare.2021.08.003.
-
Soma, M., & Lalam, S. K. (2022). The role of nicotinamide mononucleotide (NMN) in anti-aging, longevity, and its potential for treating chronic conditions. Molecular biology reports, 49(10), 9737–9748. https://doi.org/10.1007/s11033-022-07459-1.
-
Brito, S., Baek, J. M., Cha, B., Heo, H., Lee, S. H., Lei, L., Jung, S. Y., Lee, S. M., Lee, S. H., Kwak, B. M., Chae, S., Lee, M. G., & Bin, B. H. (2022). Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/Wnt signaling. Journal of dermatological science, 106(3), 159–169. https://doi.org/10.1016/j.jdermsci.2022.05.002.
-
Oblong J. E. (2014). The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA repair, 23, 59–63. https://doi.org/10.1016/j.dnarep.2014.04.005.
-
Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., D’Amico, D., Ropelle, E. R., Lutolf, M. P., Aebersold, R., Schoonjans, K., Menzies, K. J., & Auwerx, J. (2016). NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (New York, N.Y.), 352(6292), 1436–1443. https://doi.org/10.1126/science.aaf2693.
-
Long, A. N., Owens, K., Schlappal, A. E., Kristian, T., Fishman, P. S., & Schuh, R. A. (2015). Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC neurology, 15, 19. https://doi.org/10.1186/s12883-015-0272-x.
-
Yao, Z., Yang, W., Gao, Z., & Jia, P. (2017). Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neuroscience letters, 647, 133–140. https://doi.org/10.1016/j.neulet.2017.03.027.
-
Yu, M., Zheng, X., Cheng, F., Shao, B., Zhuge, Q., & Jin, K. (2022). Metformin, Rapamycin, or Nicotinamide Mononucleotide Pretreatment Attenuate Cognitive Impairment After Cerebral Hypoperfusion by Inhibiting Microglial Phagocytosis. Frontiers in neurology, 13, 903565. https://doi.org/10.3389/fneur.2022.903565.
-
Hosseini, L., Farokhi-Sisakht, F., Badalzadeh, R., Khabbaz, A., Mahmoudi, J., & Sadigh-Eteghad, S. (2019). Nicotinamide Mononucleotide and Melatonin Alleviate Aging-induced Cognitive Impairment via Modulation of Mitochondrial Function and Apoptosis in the Prefrontal Cortex and Hippocampus. Neuroscience, 423, 29–37. https://doi.org/10.1016/j.neuroscience.2019.09.037.
-
Liu, X., Dilxat, T., Shi, Q., Qiu, T., & Lin, J. (2022). The combination of nicotinamide mononucleotide and lycopene prevents cognitive impairment and attenuates oxidative damage in D-galactose induced aging models via Keap1-Nrf2 signaling. Gene, 822, 146348. https://doi.org/10.1016/j.gene.2022.146348.
-
Wang, X., Hu, X., Zhang, L., Xu, X., & Sakurai, T. (2020). Nicotinamide mononucleotide administration after sever hypoglycemia improves neuronal survival and cognitive function in rats. Brain research bulletin, 160, 98–106. https://doi.org/10.1016/j.brainresbull.2020.04.022.
-
Chandrasekaran, K., Choi, J., Arvas, M. I., Salimian, M., Singh, S., Xu, S., Gullapalli, R. P., Kristian, T., & Russell, J. W. (2020). Nicotinamide Mononucleotide Administration Prevents Experimental Diabetes-Induced Cognitive Impairment and Loss of Hippocampal Neurons. International journal of molecular sciences, 21(11), 3756. https://doi.org/10.3390/ijms21113756.
-
Kiss, T., Balasubramanian, P., Valcarcel-Ares, M. N., Tarantini, S., Yabluchanskiy, A., Csipo, T., Lipecz, A., Reglodi, D., Zhang, X. A., Bari, F., Farkas, E., Csiszar, A., & Ungvari, Z. (2019). Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. GeroScience, 41(5), 619–630. https://doi.org/10.1007/s11357-019-00074-2.
-
Wang, X., Hu, X., Yang, Y., Takata, T., & Sakurai, T. (2016). Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain research, 1643, 1–9. https://doi.org/10.1016/j.brainres.2016.04.060.
-
Tarantini, S., Valcarcel-Ares, M. N., Toth, P., Yabluchanskiy, A., Tucsek, Z., Kiss, T., Hertelendy, P., Kinter, M., Ballabh, P., Süle, Z., Farkas, E., Baur, J. A., Sinclair, D. A., Csiszar, A., & Ungvari, Z. (2019). Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox biology, 24, 101192. https://doi.org/10.1016/j.redox.2019.101192.
-
Stein, L. R., & Imai, S. (2014). Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. The EMBO journal, 33(12), 1321–1340. https://doi.org/10.1002/embj.201386917.
-
Zhao, Y., Guan, Y. F., Zhou, X. M., Li, G. Q., Li, Z. Y., Zhou, C. C., Wang, P., & Miao, C. Y. (2015). Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase-Nicotinamide Adenine Dinucleotide Cascade. Stroke, 46(7), 1966–1974. https://doi.org/10.1161/STROKEAHA.115.009216.
-
Johnson, S., Wozniak, D. F., & Imai, S. (2018). CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ aging and mechanisms of disease, 4, 10. https://doi.org/10.1038/s41514-018-0029-z.
-
Lu, L., Tang, L., Wei, W., Hong, Y., Chen, H., Ying, W., & Chen, S. (2014). Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease. Experimental and therapeutic medicine, 8(3), 943–950. https://doi.org/10.3892/etm.2014.1842.
-
Hsu, C. P., Zhai, P., Yamamoto, T., Maejima, Y., Matsushima, S., Hariharan, N., Shao, D., Takagi, H., Oka, S., & Sadoshima, J. (2010). Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation, 122(21), 2170–2182. https://doi.org/10.1161/CIRCULATIONAHA.110.958033.
-
Yamamoto, T., Byun, J., Zhai, P., Ikeda, Y., Oka, S., & Sadoshima, J. (2014). Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS one, 9(6), e98972. https://doi.org/10.1371/journal.pone.0098972.
-
Nadtochiy, S. M., Wang, Y. T., Nehrke, K., Munger, J., & Brookes, P. S. (2018). Cardioprotection by nicotinamide mononucleotide (NMN): Involvement of glycolysis and acidic pH. Journal of molecular and cellular cardiology, 121, 155–162. https://doi.org/10.1016/j.yjmcc.2018.06.007.
-
Park, J. H., Long, A., Owens, K., & Kristian, T. (2016). Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiology of disease, 95, 102–110. https://doi.org/10.1016/j.nbd.2016.07.018.
-
Martin, A. S., Abraham, D. M., Hershberger, K. A., Bhatt, D. P., Mao, L., Cui, H., Liu, J., Liu, X., Muehlbauer, M. J., Grimsrud, P. A., Locasale, J. W., Payne, R. M., & Hirschey, M. D. (2017). Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI insight, 2(14), e93885. https://doi.org/10.1172/jci.insight.93885.
-
Klimova, N., Fearnow, A., Long, A., & Kristian, T. (2020). NAD+ precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Experimental neurology, 325, 113144. https://doi.org/10.1016/j.expneurol.2019.113144.
-
Zhang, R., Shen, Y., Zhou, L., Sangwung, P., Fujioka, H., Zhang, L., & Liao, X. (2017). Short-term administration of Nicotinamide Mononucleotide preserves cardiac mitochondrial homeostasis and prevents heart failure. Journal of molecular and cellular cardiology, 112, 64–73. https://doi.org/10.1016/j.yjmcc.2017.09.001.
-
Abdellatif, M., Sedej, S., & Kroemer, G. (2021). NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation, 144(22), 1795–1817. https://doi.org/10.1161/CIRCULATIONAHA.121.056589.
-
Abdellatif, M., Trummer-Herbst, V., Koser, F., Durand, S., Adão, R., Vasques-Nóvoa, F., Freundt, J. K., Voglhuber, J., Pricolo, M. R., Kasa, M., Türk, C., Aprahamian, F., Herrero-Galán, E., Hofer, S. J., Pendl, T., Rech, L., Kargl, J., Anto-Michel, N., Ljubojevic-Holzer, S., Schipke, J., … Sedej, S. (2021). Nicotinamide for the treatment of heart failure with preserved ejection fraction. Science translational medicine, 13(580), eabd7064. https://doi.org/10.1126/scitranslmed.abd7064.
-
Wan, Y., He, B., Zhu, D., Wang, L., Huang, R., Zhu, J., Wang, C., & Gao, F. (2021). Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Archives of biochemistry and biophysics, 712, 109050. https://doi.org/10.1016/j.abb.2021.109050.
-
Jafari-Azad, A., Hosseini, L., Rajabi, M., Høilund-Carlsen, P. F., Vafaee, M. S., Feyzizadeh, S., & Badalzadeh, R. (2021). Nicotinamide mononucleotide and melatonin counteract myocardial ischemia-reperfusion injury by activating SIRT3/FOXO1 and reducing apoptosis in aged male rats. Molecular biology reports, 48(4), 3089–3096. https://doi.org/10.1007/s11033-021-06351-8.
-
Wu, K., Li, B., Lin, Q., Xu, W., Zuo, W., Li, J., Liu, N., Tu, T., Zhang, B., Xiao, Y., & Liu, Q. (2021). Nicotinamide mononucleotide attenuates isoproterenol-induced cardiac fibrosis by regulating oxidative stress and Smad3 acetylation. Life sciences, 274, 119299. https://doi.org/10.1016/j.lfs.2021.119299.
-
Yamamoto, T., Byun, J., Zhai, P., Ikeda, Y., Oka, S., & Sadoshima, J. (2014). Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS one, 9(6), e98972. https://doi.org/10.1371/journal.pone.0098972.
-
Kiss, T., Giles, C. B., Tarantini, S., Yabluchanskiy, A., Balasubramanian, P., Gautam, T., Csipo, T., Nyúl-Tóth, Á., Lipecz, A., Szabo, C., Farkas, E., Wren, J. D., Csiszar, A., & Ungvari, Z. (2019). Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. GeroScience, 41(4), 419–439. https://doi.org/10.1007/s11357-019-00095-x.
-
Whitson, J. A., Bitto, A., Zhang, H., Sweetwyne, M. T., Coig, R., Bhayana, S., Shankland, E. G., Wang, L., Bammler, T. K., Mills, K. F., Imai, S. I., Conley, K. E., Marcinek, D. J., & Rabinovitch, P. S. (2020). SS-31 and NMN: Two paths to improve metabolism and function in aged hearts. Aging cell, 19(10), e13213. https://doi.org/10.1111/acel.13213.
-
Wu, L. E., Gomes, A. P., & Sinclair, D. A. (2014). Geroncogenesis: metabolic changes during aging as a driver of tumorigenesis. Cancer cell, 25(1), 12–19. https://doi.org/10.1016/j.ccr.2013.12.005.
-
Lee MK, Cheong HS, Koh Y, Ahn KS, Yoon SS, Shin HD. Genetic Association of PARP15 Polymorphisms with Clinical Outcome of Acute Myeloid Leukemia in a Korean Population. Genet Test Mol Biomarkers. 2016;20:696–701.
-
Dollerup O.L., Christensen B., Svart M., Schmidt M.S., Sulek K., Ringgaard S., Stødkilde-Jørgensen H., Møller N., Brenner C., Treebak J.T., Jessen N. A randomized placebo-controlled clinical trial of nicotinamideriboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018;108:343–353.
-
Martens C.R., Denman B.A., Mazzo M.R., Armstrong M.L., Reisdorph N., McQueen M.B., Chonchol M., Seals D.R. Chronic nicotinamideriboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 2018;9:1286.
-
Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD Metabolism in Cancer Therapeutics. Front Oncol. 2018;8:622. Published 2018 Dec 12. doi:10.3389/fonc.2018.00622.
-
Available from https://www.biorxiv.org/content/10.1101/2020.03.21.001123v1.
-
Fania, L., Mazzanti, C., Campione, E., Candi, E., Abeni, D., & Dellambra, E. (2019). Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. International journal of molecular sciences, 20(23), 5946. https://doi.org/10.3390/ijms20235946.
-
Kusumanchi, P., Zhang, Y., Jani, M. B., Jayaram, N. H., Khan, R. A., Tang, Y., Antony, A. C., & Jayaram, H. N. (2013). Nicotinamide mononucleotide adenylyltransferase2 overexpression enhances colorectal cancer cell-kill by Tiazofurin. Cancer gene therapy, 20(7), 403–412. https://doi.org/10.1038/cgt.2013.33.
-
Yoshino, M., Yoshino, J., Kayser, B. D., Patti, G. J., Franczyk, M. P., Mills, K. F., Sindelar, M., Pietka, T., Patterson, B. W., Imai, S. I., & Klein, S. (2021). Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science (New York, N.Y.), 372(6547), 1224–1229. https://doi.org/10.1126/science.abe9985.
-
Camacho-Pereira, J., Tarragó, M. G., Chini, C., Nin, V., Escande, C., Warner, G. M., Puranik, A. S., Schoon, R. A., Reid, J. M., Galina, A., & Chini, E. N. (2016). CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell metabolism, 23(6), 1127–1139. https://doi.org/10.1016/j.cmet.2016.05.006.
-
Escande, C., Nin, V., Price, N. L., Capellini, V., Gomes, A. P., Barbosa, M. T., O’Neil, L., White, T. A., Sinclair, D. A., & Chini, E. N. (2013). Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes, 62(4), 1084–1093. https://doi.org/10.2337/db12-1139.
-
Yoshino, J., Mills, K. F., Yoon, M. J., & Imai, S. (2011). Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell metabolism, 14(4), 528–536. https://doi.org/10.1016/j.cmet.2011.08.014.
-
Choi, S. E., Fu, T., Seok, S., Kim, D. H., Yu, E., Lee, K. W., Kang, Y., Li, X., Kemper, B., & Kemper, J. K. (2013). Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging cell, 12(6), 1062–1072. https://doi.org/10.1111/acel.12135.
-
Caton, P. W., Kieswich, J., Yaqoob, M. M., Holness, M. J., & Sugden, M. C. (2011). Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia, 54(12), 3083–3092. https://doi.org/10.1007/s00125-011-2288-0.
-
Bordone, L., Motta, M. C., Picard, F., Robinson, A., Jhala, U. S., Apfeld, J., McDonagh, T., Lemieux, M., McBurney, M., Szilvasi, A., Easlon, E. J., Lin, S. J., & Guarente, L. (2006). Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS biology, 4(2), e31. https://doi.org/10.1371/journal.pbio.0040031.
-
Ramsey, K. M., Mills, K. F., Satoh, A., & Imai, S. (2008). Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging cell, 7(1), 78–88. https://doi.org/10.1111/j.1474-9726.2007.00355.x.
-
Nahle, A., Joseph, Y. D., Pereira, S., Mori, Y., Poon, F., Ghadieh, H. E., Ivovic, A., Desai, T., Ghanem, S. S., Asalla, S., Muturi, H. T., Jentz, E. M., Joseph, J. W., Najjar, S. M., & Giacca, A. (2021). Nicotinamide Mononucleotide Prevents Free Fatty Acid-Induced Reduction in Glucose Tolerance by Decreasing Insulin Clearance. International journal of molecular sciences, 22(24), 13224. https://doi.org/10.3390/ijms222413224.
-
Sheng, F., Ren, X., Dai, X., Xu, X., Dong, M., Pei, Q., Qu, J., Zhou, Z., Zhou, H., & Liu, Z. (2011). Effect of nicotinamide mononucleotide on insulin secretion and gene expressions of PDX-1 and FoxO1 in RIN-m5f cells. Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University. Medical sciences, 36(10), 958–963. https://doi.org/10.3969/j.issn.1672-7347.2011.10.005.
-
Polo, V., Saibene, A., & Pontiroli, A. E. (1998). Nicotinamide improves insulin secretion and metabolic control in lean type 2 diabetic patients with secondary failure to sulphonylureas. Acta diabetologica, 35(1), 61–64. https://doi.org/10.1007/s005920050103.
-
Liu, J., Zong, Z., Zhang, W., Chen, Y., Wang, X., Shen, J., Yang, C., Liu, X., & Deng, H. (2021). Nicotinamide Mononucleotide Alleviates LPS-Induced Inflammation and Oxidative Stress via Decreasing COX-2 Expression in Macrophages. Frontiers in molecular biosciences, 8, 702107. https://doi.org/10.3389/fmolb.2021.702107.
-
Ru, M., Wang, W., Zhai, Z., Wang, R., Li, Y., Liang, J., Kothari, D., Niu, K., & Wu, X. (2022). Nicotinamide mononucleotide supplementation protects the intestinal function in aging mice and D-galactose induced senescent cells. Food & function, 13(14), 7507–7519. https://doi.org/10.1039/d2fo00525e.
-
Cros, C., Margier, M., Cannelle, H., Charmetant, J., Hulo, N., Laganier, L., Grozio, A., & Canault, M. (2022). Nicotinamide Mononucleotide Administration Triggers Macrophages Reprogramming and Alleviates Inflammation During Sepsis Induced by Experimental Peritonitis. Frontiers in molecular biosciences, 9, 895028. https://doi.org/10.3389/fmolb.2022.895028.
-
Mateuszuk, Ł., Campagna, R., Kutryb-Zając, B., Kuś, K., Słominska, E. M., Smolenski, R. T., & Chlopicki, S. (2020). Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochemical pharmacology, 178, 114019. https://doi.org/10.1016/j.bcp.2020.114019.
-
Miao, Y., Li, X., Shi, X., Gao, Q., Chen, J., Wang, R., Fan, Y., & Xiong, B. (2021). Nicotinamide Mononucleotide Restores the Meiotic Competency of Porcine Oocytes Exposed to Ethylene Glycol Butyl Ether. Frontiers in cell and developmental biology, 9, 628580. https://doi.org/10.3389/fcell.2021.628580.
-
Bertoldo, M. J., Listijono, D. R., Ho, W. J., Riepsamen, A. H., Goss, D. M., Richani, D., Jin, X. L., Mahbub, S., Campbell, J. M., Habibalahi, A., Loh, W. N., Youngson, N. A., Maniam, J., Wong, A., Selesniemi, K., Bustamante, S., Li, C., Zhao, Y., Marinova, M. B., Kim, L. J., … Wu, L. E. (2020). NAD+ Repletion Rescues Female Fertility during Reproductive Aging. Cell reports, 30(6), 1670–1681.e7. https://doi.org/10.1016/j.celrep.2020.01.058.
-
Campbell, J. M., Mahbub, S. B., Bertoldo, M. J., Habibalahi, A., Goss, D. M., Ledger, W. L., Gilchrist, R. B., Wu, L. E., & Goldys, E. M. (2022). Multispectral autofluorescence characteristics of reproductive aging in old and young mouse oocytes. Biogerontology, 23(2), 237–249. https://doi.org/10.1007/s10522-022-09957-y.
-
Song, M., Li, Y., Zhou, Y., Yan, J., Zhou, X., Gao, Q., Miao, Y., & Xiong, B. (2022). Nicotinamide mononucleotide supplementation improves the quality of porcine oocytes under heat stress. Journal of animal science and biotechnology, 13(1), 68. https://doi.org/10.1186/s40104-022-00716-0.
-
Miao, Y., Cui, Z., Zhu, X., Gao, Q., & Xiong, B. (2022). Supplementation of nicotinamide mononucleotide improves the quality of postovulatory aged porcine oocytes. Journal of molecular cell biology, 14(4), mjac025. https://doi.org/10.1093/jmcb/mjac025.
-
Yang, L., Lin, X., Tang, H., Fan, Y., Zeng, S., Jia, L., Li, Y., Shi, Y., He, S., Wang, H., Hu, Z., Gong, X., Liang, X., Yang, Y., & Liu, X. (2020). Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD+ redox. Aging cell, 19(9), e13206. https://doi.org/10.1111/acel.13206.
-
Ma, D., Hu, L., Wang, J., Luo, M., Liang, A., Lei, X., Liao, B., Li, M., Xie, M., Li, H., Gong, Y., Zi, D., Li, X., Chen, X., & Liao, X. (2022). Nicotinamide mononucleotide improves spermatogenic function in streptozotocin-induced diabetic mice via modulating the glycolysis pathway. Acta biochimica et biophysica Sinica, 10.3724/abbs.2022099. Advance online publication. https://doi.org/10.3724/abbs.2022099.
-
Bai, S., & Sheline, C. T. (2013). NAD(+) maintenance attenuates light induced photoreceptor degeneration. Experimental eye research, 108, 76–83. https://doi.org/10.1016/j.exer.2012.12.007.
-
Lin, J. B., Kubota, S., Ban, N., Yoshida, M., Santeford, A., Sene, A., Nakamura, R., Zapata, N., Kubota, M., Tsubota, K., Yoshino, J., Imai, S. I., & Apte, R. S. (2016). NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell reports, 17(1), 69–85. https://doi.org/10.1016/j.celrep.2016.08.073.
-
Mimura, T., Kaji, Y., Noma, H., Funatsu, H., & Okamoto, S. (2013). The role of SIRT1 in ocular aging. Experimental eye research, 116, 17–26. https://doi.org/10.1016/j.exer.2013.07.017.
-
Zeng, Y., & Yang, K. (2015). Sirtuin 1 participates in the process of age-related retinal degeneration. Biochemical and biophysical research communications, 468(1-2), 167–172. https://doi.org/10.1016/j.bbrc.2015.10.139.
-
Kowluru, R. A., Santos, J. M., & Zhong, Q. (2014). Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Investigative ophthalmology & visual science, 55(9), 5653–5660. https://doi.org/10.1167/iovs.14-14383.
-
Zheng, Z., Chen, H., Li, J., Li, T., Zheng, B., Zheng, Y., Jin, H., He, Y., Gu, Q., & Xu, X. (2012). Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes, 61(1), 217–228. https://doi.org/10.2337/db11-0416.
-
Kubota, S., Kurihara, T., Ebinuma, M., Kubota, M., Yuki, K., Sasaki, M., Noda, K., Ozawa, Y., Oike, Y., Ishida, S., & Tsubota, K. (2010). Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation. The American journal of pathology, 177(4), 1725–1731. https://doi.org/10.2353/ajpath.2010.100098.
-
Chen, J., Michan, S., Juan, A. M., Hurst, C. G., Hatton, C. J., Pei, D. T., Joyal, J. S., Evans, L. P., Cui, Z., Stahl, A., Sapieha, P., Sinclair, D. A., & Smith, L. E. (2013). Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy. Angiogenesis, 16(4), 985–992. https://doi.org/10.1007/s10456-013-9374-5.
-
Meng, Y. F., Pu, Q., Dai, S. Y., Ma, Q., Li, X., & Zhu, W. (2021). Nicotinamide Mononucleotide Alleviates Hyperosmolarity-Induced IL-17a Secretion and Macrophage Activation in Corneal Epithelial Cells/Macrophage Co-Culture System. Journal of inflammation research, 14, 479–493. https://doi.org/10.2147/JIR.S292764.
-
Pu, Q., Guo, X. X., Hu, J. J., Li, A. L., Li, G. G., & Li, X. Y. (2022). Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 147, 112659. https://doi.org/10.1016/j.biopha.2022.112659.
-
Cimaglia, G., Votruba, M., Morgan, J. E., André, H., & Williams, P. A. (2020). Potential Therapeutic Benefit of NAD+ Supplementation for Glaucoma and Age-Related Macular Degeneration. Nutrients, 12(9), 2871. https://doi.org/10.3390/nu12092871.
-
Lee, D., Tomita, Y., Miwa, Y., Shinojima, A., Ban, N., Yamaguchi, S., Nishioka, K., Negishi, K., Yoshino, J., & Kurihara, T. (2022). Nicotinamide Mononucleotide Prevents Retinal Dysfunction in a Mouse Model of Retinal Ischemia/Reperfusion Injury. International journal of molecular sciences, 23(19), 11228. https://doi.org/10.3390/ijms231911228.
-
Chen, X., Amorim, J. A., Moustafa, G. A., Lee, J. J., Yu, Z., Ishihara, K., Iesato, Y., Barbisan, P., Ueta, T., Togka, K. A., Lu, L., Sinclair, D. A., & Vavvas, D. G. (2020). Neuroprotective effects and mechanisms of action of nicotinamide mononucleotide (NMN) in a photoreceptor degenerative model of retinal detachment. Aging, 12(24), 24504–24521. https://doi.org/10.18632/aging.202453.
-
Zhao, C., Li, W., Duan, H., Li, Z., Jia, Y., Zhang, S., Wang, X., Zhou, Q., & Shi, W. (2020). NAD+ precursors protect corneal endothelial cells from UVB-induced apoptosis. American journal of physiology. Cell physiology, 318(4), C796–C805. https://doi.org/10.1152/ajpcell.00445.2019.
-
Li, Y., Ma, X., Li, J., Yang, L., Zhao, X., Qi, X., Zhang, X., Zhou, Q., & Shi, W. (2019). Corneal Denervation Causes Epithelial Apoptosis Through Inhibiting NAD+ Biosynthesis. Investigative ophthalmology & visual science, 60(10), 3538–3546. https://doi.org/10.1167/iovs.19-26909.
-
Koetz, K., Bryl, E., Spickschen, K., O’Fallon, W. M., Goronzy, J. J., & Weyand, C. M. (2000). T cell homeostasis in patients with rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America, 97(16), 9203–9208. https://doi.org/10.1073/pnas.97.16.9203.
-
Fyhrquist, F., Tiitu, A., Saijonmaa, O., Forsblom, C., Groop, P. H., & FinnDiane Study Group (2010). Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. Journal of internal medicine, 267(3), 278–286. https://doi.org/10.1111/j.1365-2796.2009.02139.x.
-
Testa, R., Olivieri, F., Sirolla, C., Spazzafumo, L., Rippo, M. R., Marra, M., Bonfigli, A. R., Ceriello, A., Antonicelli, R., Franceschi, C., Castellucci, C., Testa, I., & Procopio, A. D. (2011). Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association, 28(11), 1388–1394. https://doi.org/10.1111/j.1464-5491.2011.03370.x.
-
Takeda, K., & Okumura, K. (2021). Nicotinamide mononucleotide augments the cytotoxic activity of natural killer cells in young and elderly mice. Biomedical research (Tokyo, Japan), 42(5), 173–179. https://doi.org/10.2220/biomedres.42.173.
-
Maiese, K., Chong, Z. Z., Hou, J., & Shang, Y. C. (2009). The vitamin nicotinamide: translating nutrition into clinical care. Molecules (Basel, Switzerland), 14(9), 3446–3485. https://doi.org/10.3390/molecules14093446.
-
Grahnert, A., Grahnert, A., Klein, C., Schilling, E., Wehrhahn, J., & Hauschildt, S. (2011). Review: NAD +: a modulator of immune functions. Innate immunity, 17(2), 212–233. https://doi.org/10.1177/1753425910361989.
-
Omran, H. M., & Almaliki, M. S. (2020). Influence of NAD+ as an ageing-related immunomodulator on COVID 19 infection: A hypothesis. Journal of infection and public health, 13(9), 1196–1201. https://doi.org/10.1016/j.jiph.2020.06.004.
-
Hiromatsu, Y., Yang, D., Miyake, I., Koga, M., Kameo, J., Sato, M., Inoue, Y., & Nonaka, K. (1998). Nicotinamide decreases cytokine-induced activation of orbital fibroblasts from patients with thyroid-associated ophthalmopathy. The Journal of clinical endocrinology and metabolism, 83(1), 121–124. https://doi.org/10.1210/jcem.83.1.4478.
-
Hiromatsu, Y., Sato, M., Tanaka, K., Ishisaka, N., Kamachi, J., & Nonaka, K. (1993). Inhibitory effects of nicotinamide on intercellular adhesion molecule-1 expression on cultured human thyroid cells. Immunology, 80(2), 330–332.
-
Silwal P., Shin K., Choi S., Namgung U., Lee C.Y., Heo J.-Y.-Y. Tryptophan negatively regulates IgE-mediated mast cell activation. Korean J Phys Anthropol. 2017;30:53. doi: 10.11637/kjpa.2017.30.2.53.
-
Imai, S., & Yoshino, J. (2013). The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes, obesity & metabolism, 15 Suppl 3(0 3), 26–33. https://doi.org/10.1111/dom.12171.
-
Rajman, L., Chwalek, K., & Sinclair, D. A. (2018). Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell metabolism, 27(3), 529–547. https://doi.org/10.1016/j.cmet.2018.02.011.
-
Kim, M., Seol, J., Sato, T., Fukamizu, Y., Sakurai, T., & Okura, T. (2022). Effect of 12-Week Intake of Nicotinamide Mononucleotide on Sleep Quality, Fatigue, and Physical Performance in Older Japanese Adults: A Randomized, Double-Blind Placebo-Controlled Study. Nutrients, 14(4), 755. https://doi.org/10.3390/nu14040755.
-
Liao, B., Zhao, Y., Wang, D., Zhang, X., Hao, X., & Hu, M. (2021). Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. Journal of the International Society of Sports Nutrition, 18(1), 54. https://doi.org/10.1186/s12970-021-00442-4.
-
Das, A., Huang, G. X., Bonkowski, M. S., Longchamp, A., Li, C., Schultz, M. B., Kim, L. J., Osborne, B., Joshi, S., Lu, Y., Treviño-Villarreal, J. H., Kang, M. J., Hung, T. T., Lee, B., Williams, E. O., Igarashi, M., Mitchell, J. R., Wu, L. E., Turner, N., Arany, Z., … Sinclair, D. A. (2018). Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell, 173(1), 74–89.e20. https://doi.org/10.1016/j.cell.2018.02.008.
-
Crisol, B. M., Veiga, C. B., Braga, R. R., Lenhare, L., Baptista, I. L., Gaspar, R. C., Muñoz, V. R., Cordeiro, A. V., da Silva, A., Cintra, D. E., Moura, L. P., Pauli, J. R., & Ropelle, E. R. (2020). NAD+ precursor increases aerobic performance in mice. European journal of nutrition, 59(6), 2427–2437. https://doi.org/10.1007/s00394-019-02089-z.
-
Dehhaghi, M., Panahi, H., Kavyani, B., Heng, B., Tan, V., Braidy, N., & Guillemin, G. J. (2022). The Role of Kynurenine Pathway and NAD+ Metabolism in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Aging and disease, 13(3), 698–711. https://doi.org/10.14336/AD.2021.0824.
-
Kimura, S., Ichikawa, M., Sugawara, S., Katagiri, T., Hirasawa, Y., Ishikawa, T., Matsunaga, W., & Gotoh, A. (2022). Nicotinamide Mononucleotide Is Safely Metabolized and Significantly Reduces Blood Triglyceride Levels in Healthy Individuals. Cureus, 14(9), e28812. https://doi.org/10.7759/cureus.28812.
-
Stromsdorfer, K. L., Yamaguchi, S., Yoon, M. J., Moseley, A. C., Franczyk, M. P., Kelly, S. C., Qi, N., Imai, S., & Yoshino, J. (2016). NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell reports, 16(7), 1851–1860. https://doi.org/10.1016/j.celrep.2016.07.027.
-
Uddin, G. M., Youngson, N. A., Doyle, B. M., Sinclair, D. A., & Morris, M. J. (2017). Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise. Scientific reports, 7(1), 15063. https://doi.org/10.1038/s41598-017-14866-z.
-
Wei, C. C., Kong, Y. Y., Li, G. Q., Guan, Y. F., Wang, P., & Miao, C. Y. (2017). Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway. Scientific reports, 7(1), 717. https://doi.org/10.1038/s41598-017-00851-z.
-
Sims, C. A., Guan, Y., Mukherjee, S., Singh, K., Botolin, P., Davila, A., Jr, & Baur, J. A. (2018). Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock. JCI insight, 3(17), e120182. https://doi.org/10.1172/jci.insight.120182.
-
Wei, C. C., Kong, Y. Y., Hua, X., Li, G. Q., Zheng, S. L., Cheng, M. H., Wang, P., & Miao, C. Y. (2017). NAD replenishment with nicotinamide mononucleotide protects blood-brain barrier integrity and attenuates delayed tissue plasminogen activator-induced haemorrhagic transformation after cerebral ischaemia. British journal of pharmacology, 174(21), 3823–3836. https://doi.org/10.1111/bph.13979.
-
Zhang, X. Q., Lu, J. T., Jiang, W. X., Lu, Y. B., Wu, M., Wei, E. Q., Zhang, W. P., & Tang, C. (2015). NAMPT inhibitor and metabolite protect mouse brain from cryoinjury through distinct mechanisms. Neuroscience, 291, 230–240. https://doi.org/10.1016/j.neuroscience.2015.02.007.
-
Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., Machado De Oliveira, R., Leid, M., McBurney, M. W., & Guarente, L. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature, 429(6993), 771–776. https://doi.org/10.1038/nature02583.
-
Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M., & Puigserver, P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature, 434(7029), 113–118. https://doi.org/10.1038/nature03354.
-
Yang, F., Vought, B. W., Satterlee, J. S., Walker, A. K., Jim Sun, Z. Y., Watts, J. L., DeBeaumont, R., Saito, R. M., Hyberts, S. G., Yang, S., Macol, C., Iyer, L., Tjian, R., van den Heuvel, S., Hart, A. C., Wagner, G., & Näär, A. M. (2006). An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature, 442(7103), 700–704. https://doi.org/10.1038/nature04942.
-
Assiri, M. A., Ali, H. R., Marentette, J. O., Yun, Y., Liu, J., Hirschey, M. D., Saba, L. M., Harris, P. S., & Fritz, K. S. (2019). Investigating RNA expression profiles altered by nicotinamide mononucleotide therapy in a chronic model of alcoholic liver disease. Human genomics, 13(1), 65. https://doi.org/10.1186/s40246-019-0251-1.
-
Guarino M, Dufour JF. Nicotinamide and NAFLD: Is There Nothing New Under the Sun?. Metabolites. 2019;9(9):180. Published 2019 Sep 10. https://doi:10.3390/metabo9090180.
-
Wang S, Wan T, Ye M, et al. Nicotinamideriboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol. 2018;17:89-98. https://doi:10.1016/j.redox.2018.04.006.
-
Zong, Z., Liu, J., Wang, N., Yang, C., Wang, Q., Zhang, W., Chen, Y., Liu, X., & Deng, H. (2021). Nicotinamide mononucleotide inhibits hepatic stellate cell activation to prevent liver fibrosis via promoting PGE2 degradation. Free radical biology & medicine, 162, 571–581. https://doi.org/10.1016/j.freeradbiomed.2020.11.014.
-
Luo, C., Ding, W., Yang, C., Zhang, W., Liu, X., & Deng, H. (2022). Nicotinamide Mononucleotide Administration Restores Redox Homeostasis via the Sirt3-Nrf2 Axis and Protects Aged Mice from Oxidative Stress-Induced Liver Injury. Journal of proteome research, 21(7), 1759–1770. https://doi.org/10.1021/acs.jproteome.2c00167.
-
Guan, Y., Wang, S. R., Huang, X. Z., Xie, Q. H., Xu, Y. Y., Shang, D., & Hao, C. M. (2017). Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. Journal of the American Society of Nephrology : JASN, 28(8), 2337–2352. https://doi.org/10.1681/ASN.2016040385.
-
Jia, Y., Kang, X., Tan, L., Ren, Y., Qu, L., Tang, J., Liu, G., Wang, S., Xiong, Z., & Yang, L. (2021). Nicotinamide Mononucleotide Attenuates Renal Interstitial Fibrosis After AKI by Suppressing Tubular DNA Damage and Senescence. Frontiers in physiology, 12, 649547. https://doi.org/10.3389/fphys.2021.649547.
-
Hasegawa, K., Sakamaki, Y., Tamaki, M., & Wakino, S. (2022). Nicotinamide mononucleotide ameliorates adriamycin-induced renal damage by epigenetically suppressing the NMN/NAD consumers mediated by Twist2. Scientific reports, 12(1), 13712. https://doi.org/10.1038/s41598-022-18147-2.
-
Yasuda, I., Hasegawa, K., Sakamaki, Y., Muraoka, H., Kawaguchi, T., Kusahana, E., Ono, T., Kanda, T., Tokuyama, H., Wakino, S., & Itoh, H. (2021). Pre-emptive Short-term Nicotinamide Mononucleotide Treatment in a Mouse Model of Diabetic Nephropathy. Journal of the American Society of Nephrology : JASN, 32(6), 1355–1370. https://doi.org/10.1681/ASN.2020081188.
-
Chen, Y., Liang, Y., Hu, T., Wei, R., Cai, C., Wang, P., Wang, L., Qiao, W., & Feng, L. (2017). Endogenous Nampt upregulation is associated with diabetic nephropathy inflammatory-fibrosis through the NF-κB p65 and Sirt1 pathway; NMN alleviates diabetic nephropathy inflammatory-fibrosis by inhibiting endogenous Nampt. Experimental and therapeutic medicine, 14(5), 4181–4193. https://doi.org/10.3892/etm.2017.5098.
Share

Discover the Latest Trends in Wellness
At Trending Wellness Store, we stay abreast of the latest developments in health and wellness to bring you innovative products that align with current trends. Our commitment to continuous learning ensures that we provide you with the best options available.

